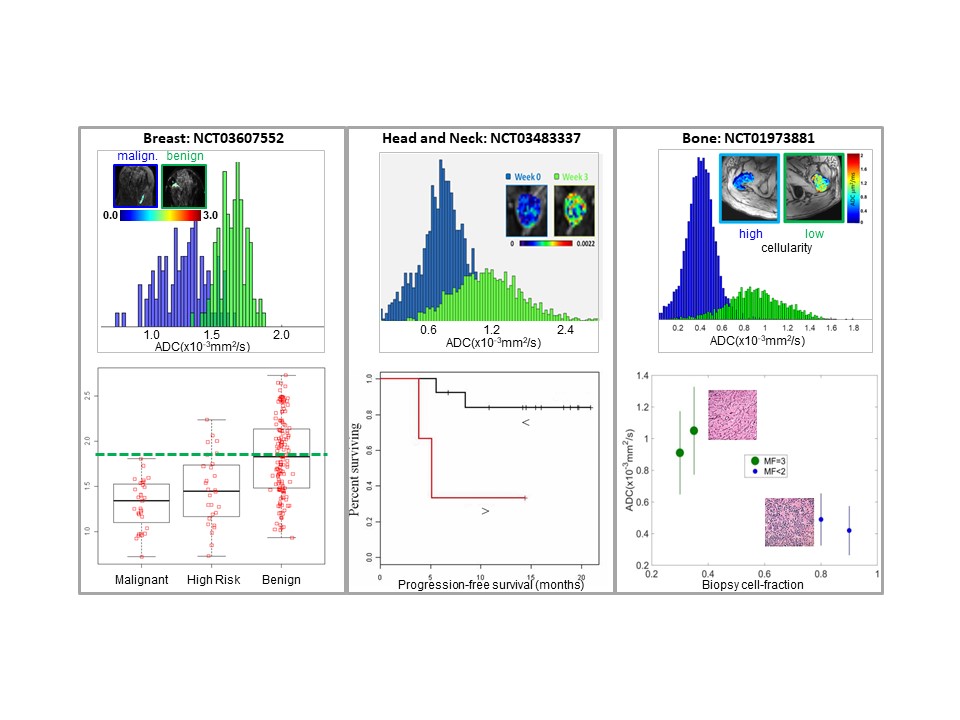
Multi-center clinical trials increasingly utilize quantitative diffusion imaging (DWI) to aid in patient management and treatment response assessment for translational oncology applications. A major source of systematic bias in diffusion was discovered originating from platform-dependent gradient hardware. Left uncorrected, these biases confound quantitative diffusion metrics used for characterization of tissue pathology and treatment response leading to inconclusive findings, and increasing the requisite subject numbers and trial cost. We have developed technology to mitigate systematic diffusion mapping bias that exists on MRI scanners and are in process of deploying this technology for multi-center clinical trials. Another major source of variance and bottleneck in high-throughput analysis of quantitative diffusion maps is segmentation of tumor/tissue volume of interest (VOI) based on intensities and patterns on multi-contrast MR image datasets, as well as reliable assessment of longitudinal change with disease progression or response to treatment. Our goal is development/trial/application AI algorithms for robust (semi-) automated VOI definition in analysis of multi-dimensional MR datasets for oncology trials.